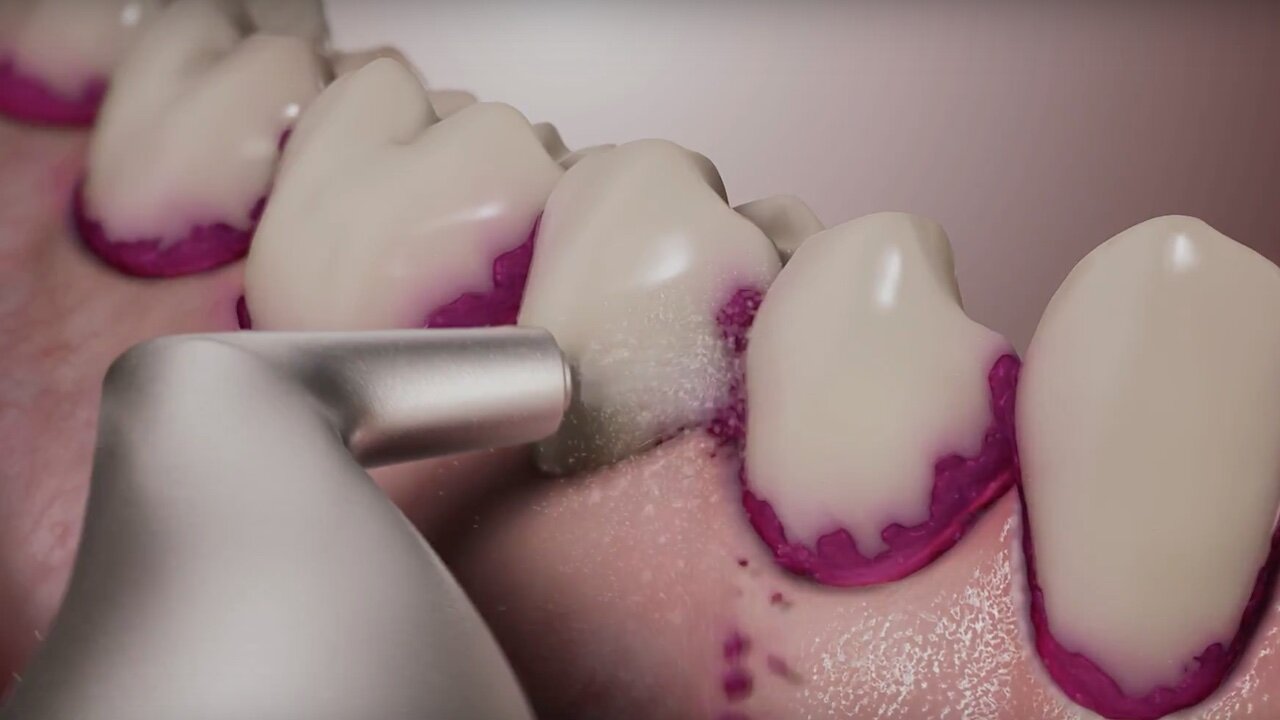
Airpolishing or even Air-Flowing is used successfully not only for prevention in many dental practices, but also in periodontal therapy. The procedure is not yet included in the current guidelines for treating periodontitis, however. Prof. em. Dr. Ulrich Schlagenhauf, the internationally renowned periodontist and former head of the Periodontology Department at the University Hospital of Würzburg, has conducted research on periodontitis and periodontal therapy for decades.
A new study by the Würzburg team – Prof. Schlagenhauf, Dr. Jeanine V. Hess, Dr. Peggy Stölzel, Dr. Imme Haubitz and Prof. Yvonne Jockel-Schneider – was published in December 2021 in the „Journal of Periodontology“ in which a two-stage subgingival instrumentation scheme involving Air-Flowing was investigated with regard to attachment gain (Schlagenhauf, U, Hess, JV, Stölzel, P, Haubitz, I, Jockel- Schneider, Y. Impact of a two-stage subgingival instrumentation scheme involving air polishing on attachment gain after active periodontal therapy. J Periodontol 2022; 00 1–10. https://doi.org/10.1002/JPER.21-0351, Open Access). Air-Flowing is the concept developed by the EMS company as a synergy of the Airflow ProphylaxisMaster, the corresponding handpieces and the erythritol-based Airflow Plus powder. In the interview with Dr. Klaus-Dieter Bastendorf, Esslingen, and Uwe Meyer, Member of the Board of Directors at EMS (Nyon/Switzerland), Schlagenhauf spoke in the spring of 2022 about the results of the study and the possible applications of airpolishing in periodontal therapy. In order to make the wide ranging basic principles and sources of his statements accessible to readers interested in science, Prof. Schlagenhauf has kindly supplemented the sources cited in the study to speed up access for the interview.
Prof. Schlagenhauf, thank you very much for taking the time for this interview. Have you been able to confirm the hypothesis of your work—that granulation tissue in deep infrabony pockets is able to autoregenerate?6,7,8,9,10

Foto: Quintessence News
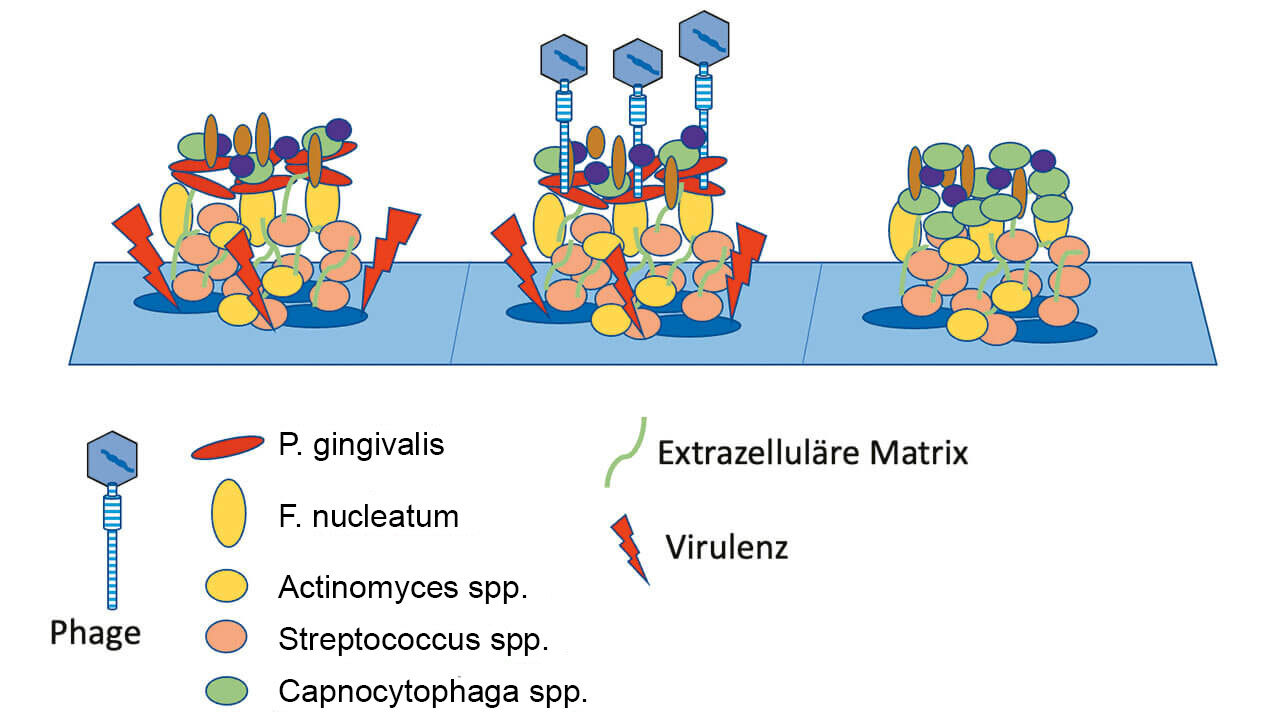
In a controlled clinical study, we have now investigated this hypothesis in 44 patients with untreated Stage III / Grade B or C periodontitis. In the test group, anti-infective therapy in the subgingival area was performed solely with the Airflow Prophylaxis Master (powder jet unit) using the low-abrasive EMS Plus powder.11-18 This meant that the existing subgingival calculus was purposefully cleaned and polished only of adherent soft bacterial biofilms, while calculus was not removed. In the control group, however, a generally accepted cleaning method using scalers and curettes according to current guidelines was used.
As scaling and root planing represent the current standard for the therapy of periodontal diseases, it was necessary, for ethical reasons, to remove the subgingival calculus by scaling and root planing in the test group after an initial healing interval of 28 days. From this point on, the root surfaces in both groups were therefore comparably free of mineralized and soft bacterial deposits. However, our assumption that initially limiting therapy to the less invasive subgingival powder jet cleaning would result in an improved gain in attachment could not be substantiated after six months at the end of the study. Statistically, no advantage of our two-stage cleaning approach could be observed in terms of a gain in attachment.
However, a detailed analysis of the healing process over time revealed that healing of periodontal inflammation during the first 28 days occurred without significant differences in both groups. In other words, although subgingival calculus was not removed in the test group, and the pockets were cleaned only of bacterial biofilm, the decrease in subgingival inflammation, expressed as a decrease in the number of pockets bleeding on probing, was equally pronounced in both experimental groups. This in itself is a remarkable observation, as many periodontists still attach primary importance to intensive mechanical instrumentation of the root surfaces and thus to the complete mechanical removal of cementum covered with subgingival calculus and plaque.
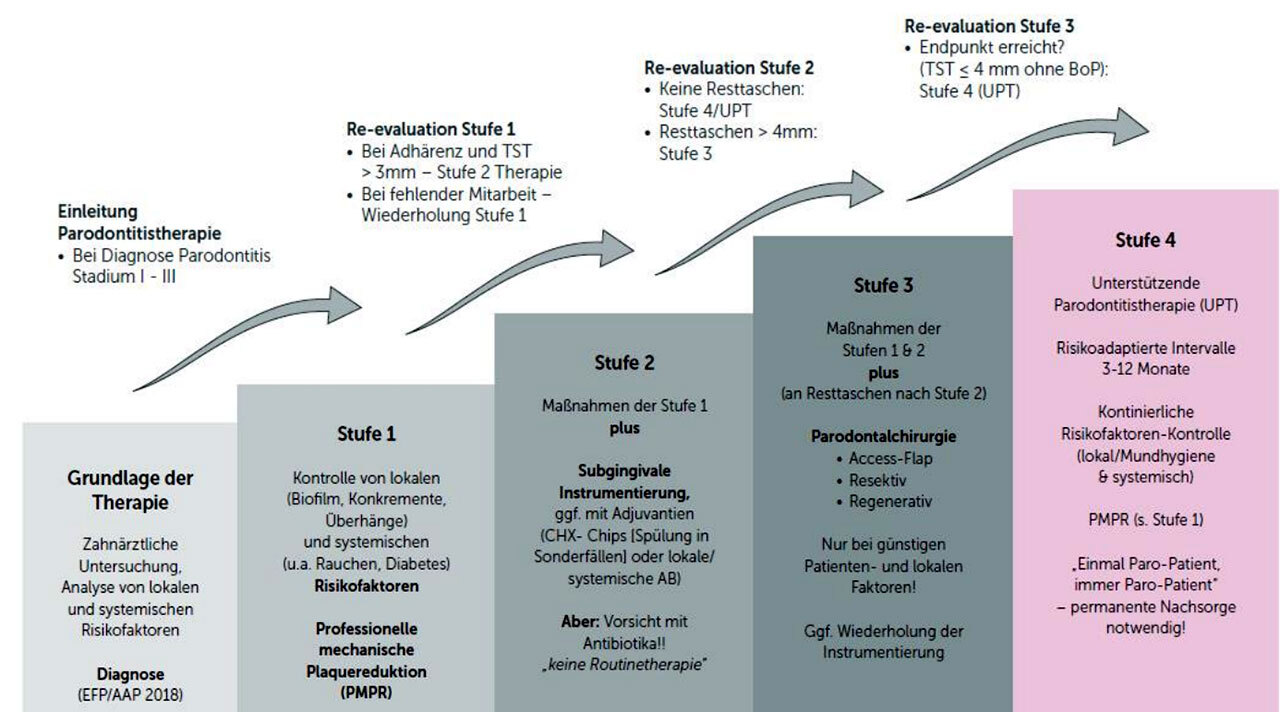
The new S3 periodontitis guideline from the German Society of Periodontology (DG PARO) based on the EFP guidelines of 2018 also commented on subgingival instrumentation “scaling versus non-scaling”. The literature available on this subject is rather sparse. Have you not made a significant contribution with your work by the 28-day results?
Schlagenhauf: Yes, I certainly think our data will provide new food for thought. However, as our study has only been published recently, the resulting findings could not yet be incorporated into current guidelines.
The results, especially after 28 days, show that Airflow alone as an initial therapy achieves the same results as classical SRP. Would it be possible for the new Perioflow system—developed specifically for deep pockets with a pocket depth of more than four millimeters—to achieve better results?
Schlagenhauf: This is certainly conceivable in principle and represents a possible weakness of our study design. However, inserting the nozzle attachment into the periodontal pocket can also potentially lead to additional mechanical stress on the inflamed soft tissue, which is why in the current study we only applied the Plus powder paragingivally, i.e., from outside the pockets into the pockets.
A longer-term study is planned
In periodontology, antibiotics are often used as an adjuvant in deep pockets—would a further group with adjunctive antibiotic therapy have been indicated?
Schlagenhauf: That's an important point. The patients in our study all presented with multiple deep periodontal pockets with a probing depth of ≥ 6 millimeters. They thus represented a study cohort with high disease intensity, in which the adjuvant use of systemic antibiotics concomitant with mechanical pocket cleaning significantly improves the healing of periodontal inflammation, which otherwise can usually be satisfactorily controlled only by a combination of closed and surgical open curettage.
Would it make sense to study the Airflow group comparatively over a longer period of time, assuming the ethics committee would agree to such an approach? How long would such a study have to continue to obtain meaningful results?
Schlagenhauf: Our analysis of the healing process in both experimental groups showed that cleaning and debridement of the subgingival root surfaces with curettes, as was also performed in the test group after 28 days, did not accelerate healing in the test group, but rather inhibited it when compared to the control group. The reasons for this are not clear.
We know that periodontal healing processes progress slowly and that the remodeling processes in the periodontium are often not completely finished, even after a year. Inevitable traumatic mechanical treatment of the subgingival root surfaces at this early stage of healing may have had a significant adverse effect on these processes.
In planned follow-up studies, we therefore intend to extend the interval between minimally-invasive Airflow powder jet cleaning and subsequent mechanical removal of mineralized biofilm to three months. As the initial soft tissue healing of the patients treated only with powder jet cleaning in the current study was absolutely the same and inconspicuous compared to the control group treated with scaling and root planing, as mentioned before, such an approach would certainly not pose any relevant health risk to the patients participating in the study.
Consider powder jet application when updating guidelines
There are a number of publications demonstrating that Airflow is useful as an adjuvant in anti-infective therapy. However, do the EFP and the DG PARO not mention the procedure in their guidelines?
Schlagenhauf: It is indeed unusual that, despite a broad available data base, neither Airflow nor other powder jet cleaning procedures are mentioned by name in the guidelines issued by the EFP and DG PARO, while at the same time the clinical benefits of other alternative therapeutic procedures, such as the adjuvant application of lasers or photodynamic therapy, have been thoroughly evaluated in meta- analyses. Unfortunately, I don't know the reasons for this. However, I will actively work toward changing this in the regularly scheduled updates to the guidelines.
The 28-day results could also have an impact on the long-term approach to supportive periodontal therapy. Is it conceivable in the future that supra- and subgingival biofilm management with Airflow will be possible every three months without the use of hand or machine instruments?
Schlagenhauf: Cleaning periodontal pockets using Airflow powder jet cleaning alone as part of supportive periodontal therapy is at least conceivable based on our study results and would certainly be an attractive alternative to the traditional procedure from both a cost and time perspective. However, the equivalence of this approach should first be validated in further appropriate controlled clinical studies.
You did not collect patient and practitioner satisfaction data. Can you give us your subjective impression on these points?
Schlagenhauf: Actually, we did not systematically collect data on this issue, but documented only the possible occurrence of unexpected side effects of both investigated therapeutic procedures. There was no corresponding feedback from either the patients initially treated with Airflow or the conventionally treated control patients. However, various patients from the test group spontaneously told us at initial follow-up appointments that they were pleasantly surprised by the low level of pain caused by powder jet cleaning. In my opinion, powder cleaning is also an attractive option for the practitioner due to its easy handling and the noticeably reduced time required as compared to the conventional procedure.
Proven equivalence or superiority is the benchmark
What do you think of the modified statement by Listl and Birch, 2013: “If integration of a new therapy is being considered, then should it be superior in terms of clinical and microbiological outcomes or substance conservation and/or offer additional relevant aspects, such as patient and practitioner satisfaction, time savings, or cost-effectiveness.”?
Schlagenhauf: In my opinion, the decisive criterion for replacing proven therapeutic methods through innovative methods should always be the superiority, or at least equivalence, of the treatment outcome, as proven by the results of controlled clinical studies. New procedures with clinically equivalent efficacy may then be of particular interest if their use is more cost-effective or subjectively more agreeable to patients.
From this point of view, would you recommend the Airflow procedure as the standard in anti-infective therapy and supportive periodontal therapy?
Schlagenhauf: This powder jet cleaning using low-abrasive powders such as glycine or erythritol has been an integral part of the periodontological treatment spectrum at Würzburg University's Periodontology Department for more than 20 years, and is routinely applied in almost all of our patients. Provided it is applied as intended, I can therefore recommend it without reservations for use in anti-infective therapy and supportive periodontal therapy.
German Version is available here./Deutsche Fassung hier.
Literatur
Original Publication: Schlagenhauf, U, Hess, JV, Stölzel, P, Haubitz, I, Jockel-Schneider, Y. Impact of a two-stage subgingival instrumentation scheme involving air polishing on attachment gain after active periodontal therapy. J Periodontol 2022; 00 1– 10. https://doi.org/10.1002/JPER.21-0351
References
1. Jones WA, O’Leary TJ. The effectiveness of in vivo root planning in removing bacterial endotoxin from the roots of periodontally involved teeth. J Periodontol. 1978;49:337-342.
2. O’Leary TJ. The impact of research on scaling and root planing. J Periodontol. 1986;57:69-75.
3. Suvan J, Leira Y, Moreno Sancho FM, Graziani F, Derks J, Tomasi C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J Clin Periodontol. 2020;47(Suppl 22):155-175.
4. Zappa U, Cadosch J, Simona C, Graf H, Case D. In vivo scaling and root planing forces. J Periodontol. 1991;62:335-340.
5. Rupf S, Brader I,Vonderlind D, et al. In vitro, clinical, and microbiological evaluation of a linear oscillating device for scaling and root planing. J Periodontol. 2005;76:1942-1949.
6. Saygin NE, Giannobile WV, Somerman MJ. Molecular and cell biology of cementum. Periodontol 2000. 2000;24:73-98.
7. Narayanan AS, Bartold PM. Biochemistry of periodontal connective tissues and their regeneration: a current perspective. Connect Tissue Res. 1996;34:191-201.
8. Kaufmann ME, Wiedemeier DB, Zellweger U, Solderer A, Attin T, Schmidlin PR. Gingival recession after scaling and root planing with or without systemic metronidazole and amoxicillin: a re-review. Clin Oral Investig. 2020;24: 1091-1100.
9. Lindhe J, Nyman S. Scaling and granulation tissue removal in periodontal therapy. J Clin Periodontol. 1985;12:374-388.
10. Richardson AC, Chadroff B, Bowers GM. The apical location of calculus within the intrabony defect. J Periodontol. 1990;61:118-122.
11. Cobb CM, Daubert DM, Davis K, et al. Consensus conference findings on supragingival and subgingival air polishing. Compend Contin Educ Dent. 2017;38:e1-e4.
12. Nascimento GG, Leite FRM, Pennisi PRC, Lopez R, Paranhos LR. Use of air polishing for supra- and subgingival biofilm removal for treatment of residual periodontal pockets and supportive periodontal care: a systematic review. Clin Oral Investig. 2021;25:779-795.
13. Tsang YC, Corbet EF, Jin LJ. Subgingival glycine powder airpolishing as an additional approach to nonsurgical periodontal therapy in subjects with untreated chronic periodontitis. J Periodontal Res. 2018;53:440-445.
14. Petersilka GJ. Subgingival air-polishing in the treatment of periodontal biofilm infections. Periodontol 2000. 2011;55:124-142.
15. Petersilka GJ, Tunkel J, Barakos K, Heinecke A, Haberlein I, Flemmig TF. Subgingival plaque removal at interdental sites using a low-abrasive air polishing powder. J Periodontol. 2003;74:307-311.
16. Muller N, Moene R, Cancela JA, Mombelli A. Subgingival airpolishing with erythritol during periodontal maintenance: randomized clinical trial of twelve months. J Clin Periodontol. 2014;41:883-889.
17. Hagi TT, Hofmanner P, Salvi GE, Ramseier CA, Sculean A. Clinical outcomes following subgingival application of a novel erythritol powder by means of air polishing in supportive
periodontal therapy: a randomized, controlled clinical study. Quintessence Int. 2013;44:753-761.
18. Moene R, Decaillet F, Andersen E, Mombelli A. Subgingival plaque removal using a new air-polishing device. J Periodontol 2010;81:79-88.
19. O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38.
20. Schulz KF, Altman DG, Moher D. Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. PLoS Med. 2010;7:e1000251.
21. Cobb CM, Sottosanti JS.Are-evaluation of scaling and root planing. J Periodontol. 2021;92:1370-1378.
22. Hammerle CH, Giannobile WV. Working Group 1 of the European Workshop on P. Biology of soft tissue wound healing and regeneration–consensus report of Group 1 of the 10th European Workshop on Periodontology. J Clin Periodontol. 2014;41(Suppl 15):S1-S5.
23. Eickholz P, Koch R, Kocher T, et al. Clinical benefits of systemic amoxicillin/metronidazole may depend on periodontitis severity and patients’ age: an exploratory sub-analysis of the ABPARO trial. J Clin Periodontol. 2019;46:491-501.